Experiments with mokume gane
For my end of year project in AP chemistry, we were assigned to explore any topic in chemistry. I decided to study copper-nickel mokume gane. Mokume gane is a Japanese technique of layering metals, very similar to damascus steel. I wanted to study mokume gane by creating my own sample of it.
My rough plan to create mokume gane was as follows: clean the quarters with acetone in order to remove any oils or impurities that would prevent a successful forge weld; stack up ten quarters and heat them until yellow hot with a MAPP gas torch, a proprietary mixture of gases that burns at 5300F, significantly higher than the melting temperature of both copper and nickel; hammer the quarters on an anvil so that they forge together and form metallic bonds, which constitutes the creation of a single billet; remove most of the oxide layer on the outside using a file and sandpaper; take several miscellaneous cuts from the metal in order to expose the pattern.

On my first attempt, I placed the stack of quarters on an anvil before heating them up with the torch. I got them to about a red hot heat before they appeared to stop heating up any further. I then hammered them but they did not stick together. I believe that heating them up on a metal anvil stopped them from heating up to forging temperature because the metal anvil was wicking away heat faster than I could provide it with a torch.

Before my second attempt, I was under the impression that my first attempt did not work because of the sudden impact of the hammer. This time I used sandpaper to clean off the surfaces more thoroughly than in my first attempt and heated it up while in a vise instead of on the anvil. My goal was to slowly press the quarters together instead of using sudden large impacts as I did with the hammer. Unfortunately, the quarters only reached red hot. When I released them from the vise, the instantly separated from each other. For my third attempt, I realized that the low temperature was my issues. To combat this, I heated them up on a brick because it has a much lower thermal conductivity than the cast iron vids. This lower thermal conductivity allowed me to heat the stack up to a yellow-hot state. When I hit them together with a hammer, they instantly stuck together. I also continued to forge them slightly flatter on my anvil since they were already up to forging temperature. I then filed off all of the oxides created in forging and took several cuts as well as drilled a hole to reveal the pattern inside. Minor separations between the quarters are present in the final product. This is likely due to me not cleaning the quarters carefully enough or not hitting them hard enough; regardless, they stuck together enough to etch them.
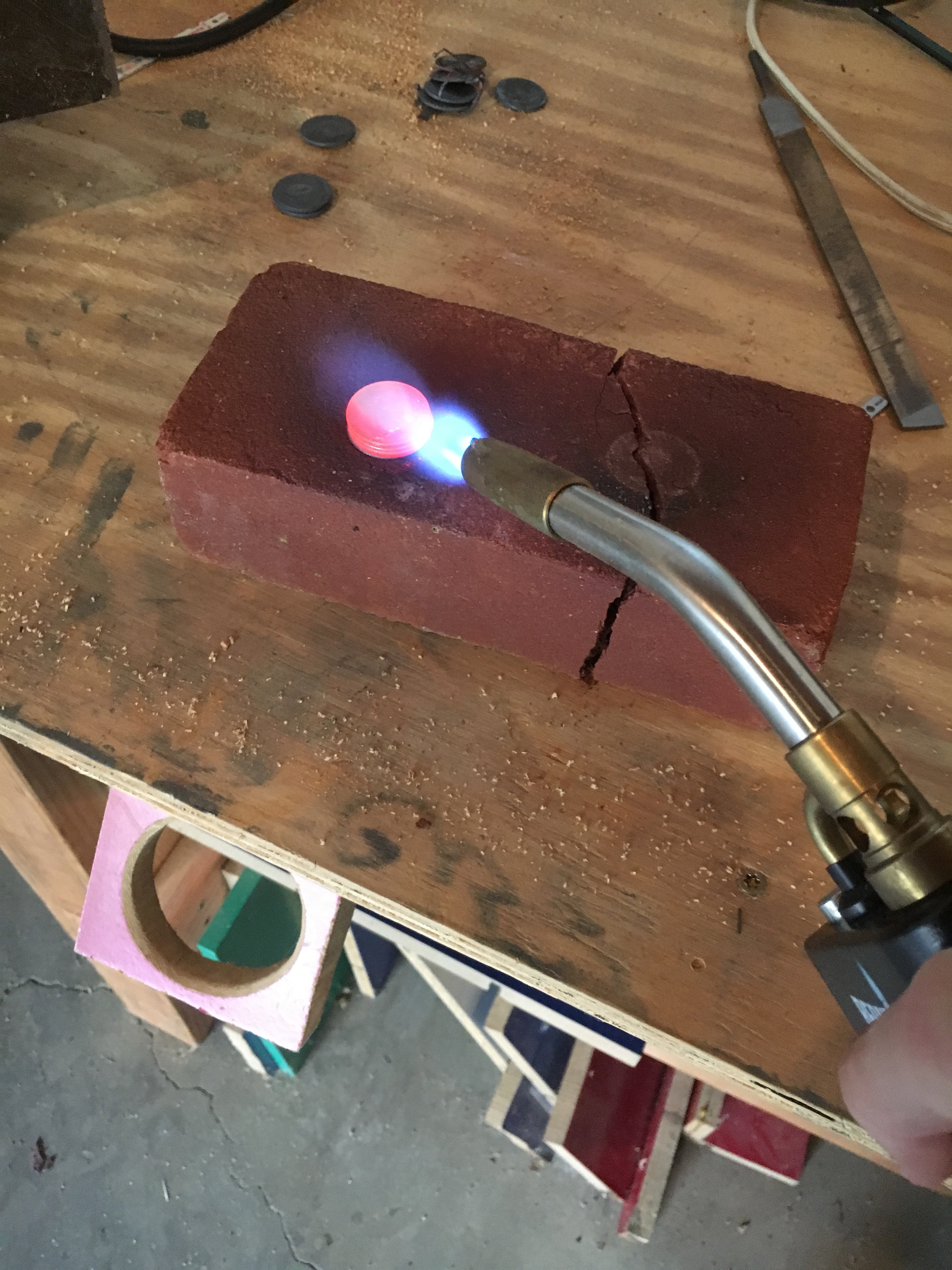

I placed the billet in 6M nitric acid to etch the away the oxide layers and ideally remove some metal from the surface of the billet. The plan was to utilize the differing reactivities of nickel and copper to create different depths of etch in the billet. Nickel has a higher reactivity than copper, so theoretically it should have etched deeper. In actuality, the two metals etched to nearly the same depth, but examination under a microscope revealed that the copper actually etched slightly deeper than the nickel, opposite to my hypothesis.
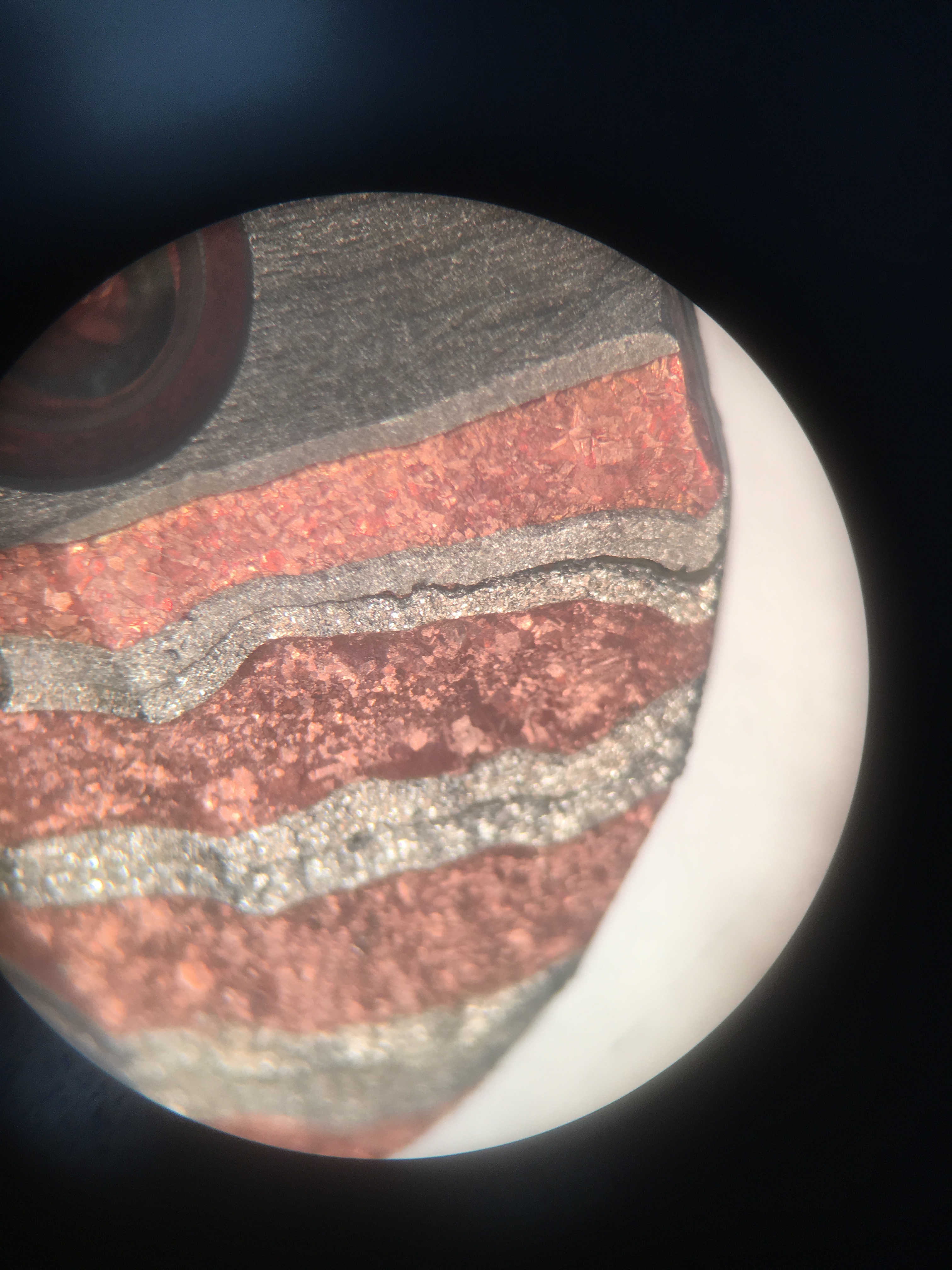
I have several candidates reasons for this.
First, it is possible that although copper has a higher reactivity than nickel, an unknown reaction between copper and sulfuric acid could have occurred, causing the reaction rate to be fast than that between nickel and sulfuric acid.
Second, the two metals both formed an oxide surface layer in the air as all metals do. The nickel oxide layer could have had a lower rate of reaction with sulfuric acid than the copper oxide layer. This would have caused the nickel metal to be shielded from the acid, causing it to etch more slowly than the copper.
Third, we noticed the solution slowly became increasingly blue throughout the reaction, causing us to believe that it was nearly saturated with the metals. Nickel was exposed on both the top and bottom of the billet while copper was much less exposed. This could have caused a significant amount of nickel to already be in solution before I removed the billet from the acid. Since a greater amount of nickel would have been present in its aqueous form, it would have lowered the rate of reaction for dissolution of nickel, causing copper to dissolve more quickly.
